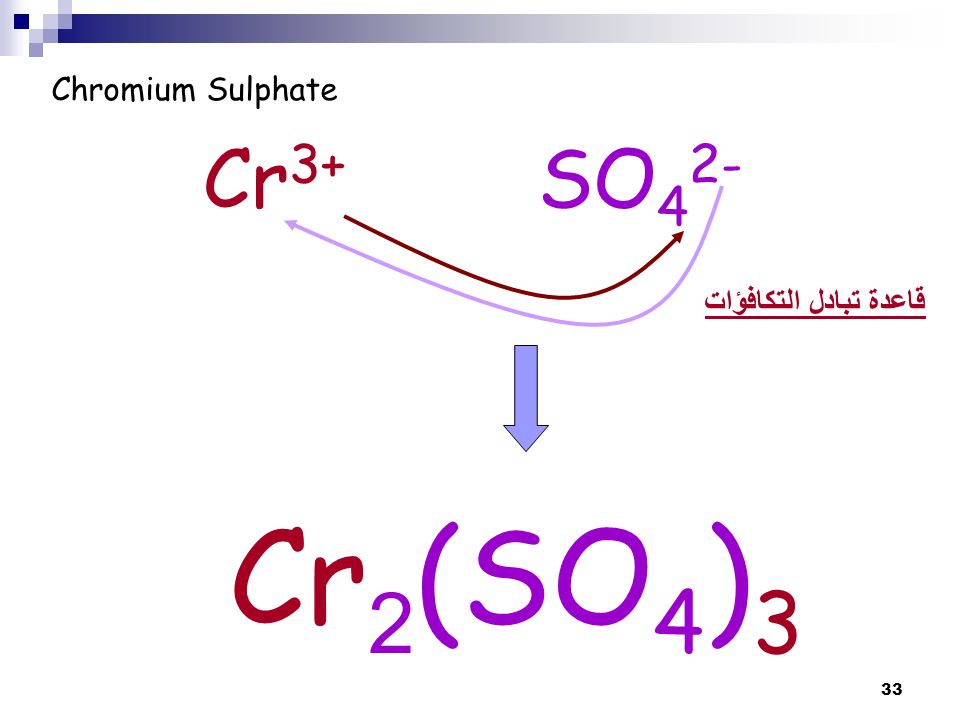
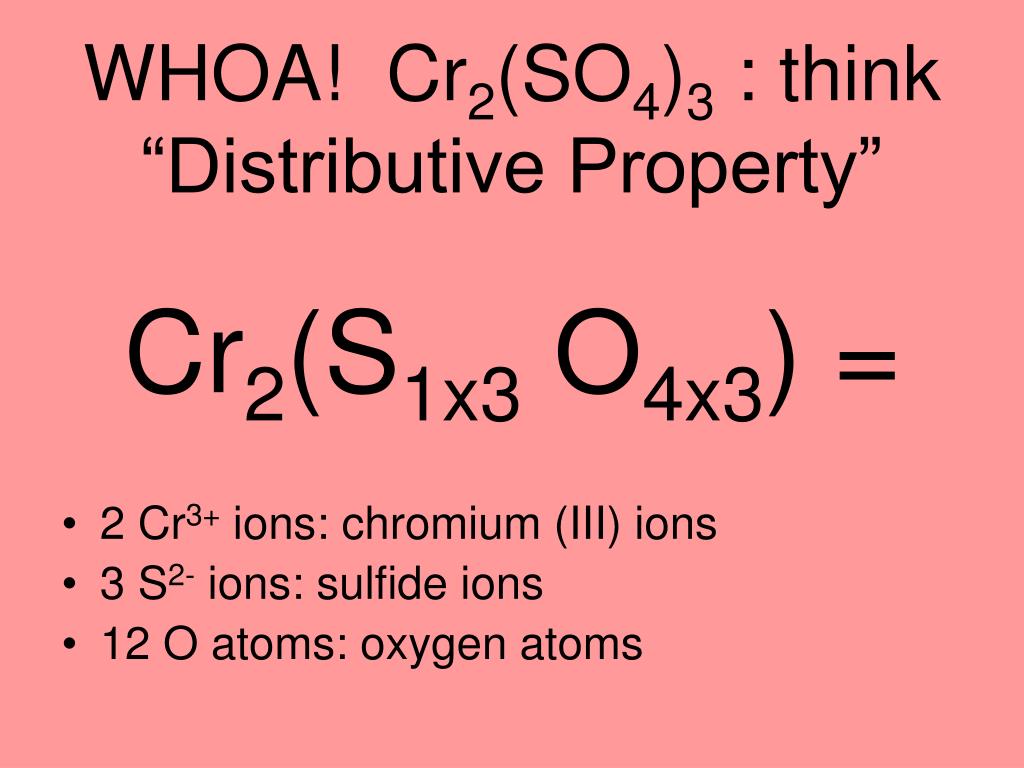
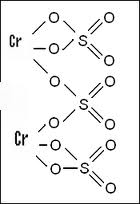
Balance the following equations by oxidation number method 1. K2Cr2O7 + KI + H2SO2 → K2SO4 + Cr2(SO4)3 + I2 + H2O - Sarthaks eConnect | Largest Online Education Community
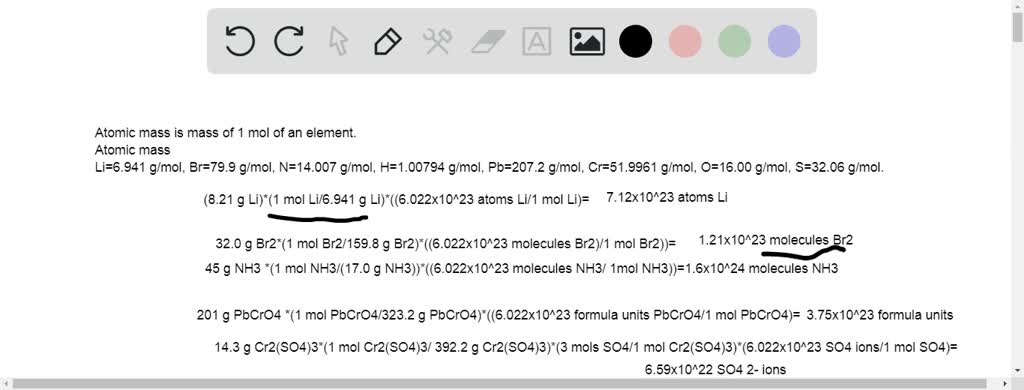
SOLVED:Calculate the following. number of atoms in 8.21 \mathrm{~g} \mathrm{Li} number of atoms in 32.0 \mathrm{~g} \mathrm{Br}_{2} number of molecules in 45 \mathrm{~g} \mathrm{NH}_{3} number of formula units in 201 \mathrm{~g} \mathrm{PbCrO}_{4}

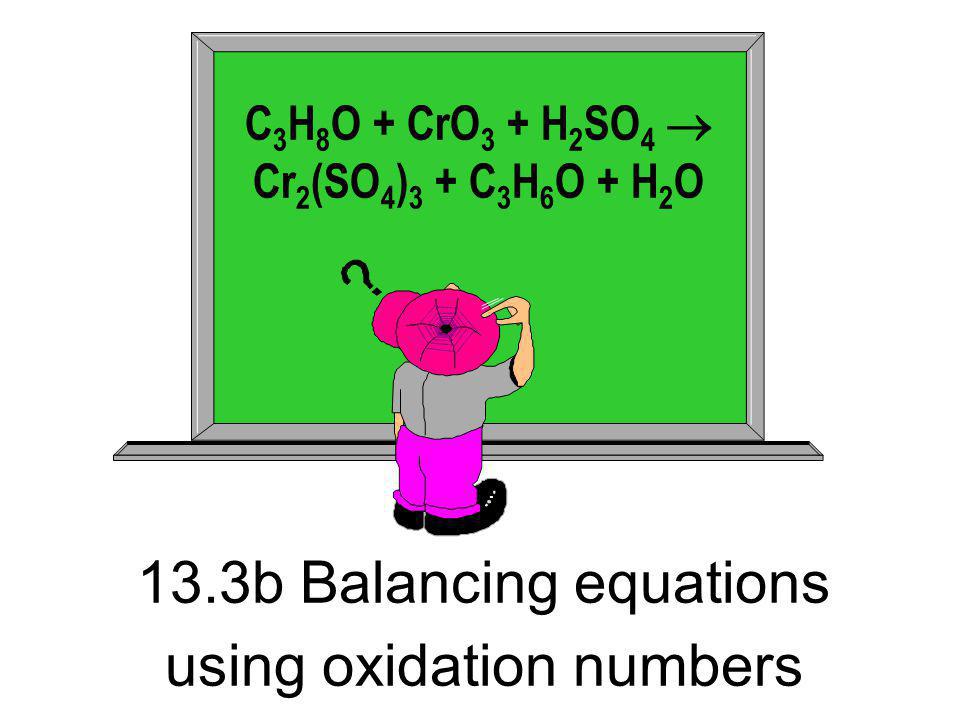
Balance the following equation by oxidation number method. PbCrO4 + H2SO4 + FeSO4→ Fe2(SO4)3 + PbSO4 + Cr2(SO4)3 + H2O
![The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O](https://haygot.s3.amazonaws.com/questions/1305019_698125_ans_e9f07e9b3a3e45088aea1d3aa5aa36ea.jpg)
The equivalent weight of Cr2(SO4)3 [mot wt. = M] in the following reaction is Cr2(SO4)3 + H2O2 + NaOH → Na2CrO4 + Na2SO4 + H2O
Balance the following equations by oxidation number method 1. K2Cr2O7 + KI + H2SO2 → K2SO4 + Cr2(SO4)3 + I2 + H2O - Sarthaks eConnect | Largest Online Education Community